We are renaming our Quality solution as part of the Egnyte for Life Sciences platform to Controlled Document Management. This will better reflect the intention and capabilities of the solution.
This change will be effective around September 14th, 2022 on all accounts with the Quality app. The product name change has no impact on the existing functionality of the product. The abbreviated name Controlled Documents will be used within some areas of the product. Refer to the screenshots below to find where users can expect to see the new product name.
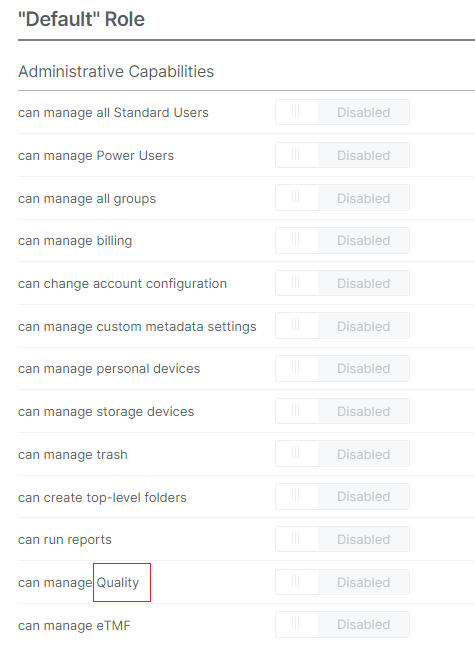
Applications Menu
Quality will be renamed to Controlled Documents.
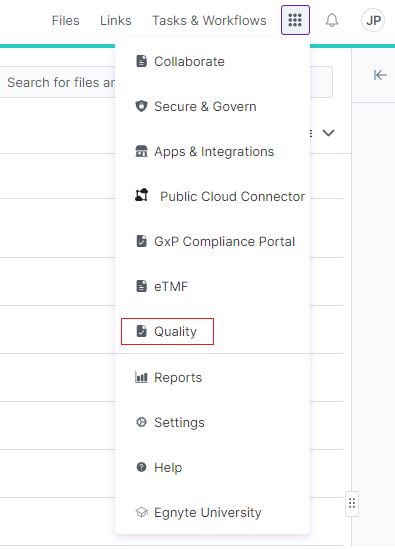
Application Home Page
Egnyte for Life Sciences Quality will be renamed to Controlled Document Management.
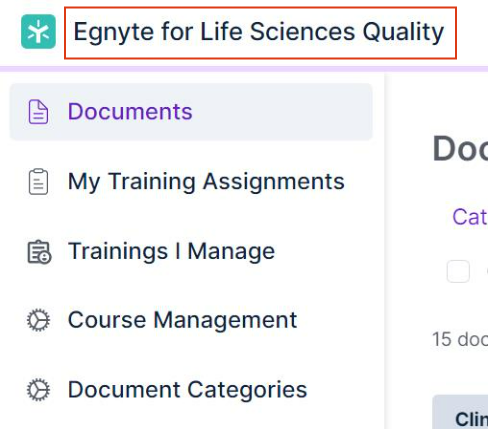
Audit Reports
Quality Documents Reports will be renamed to Controlled Documents Report.
Quality Documents Courses Reports will be renamed to Controlled Documents Courses Report.
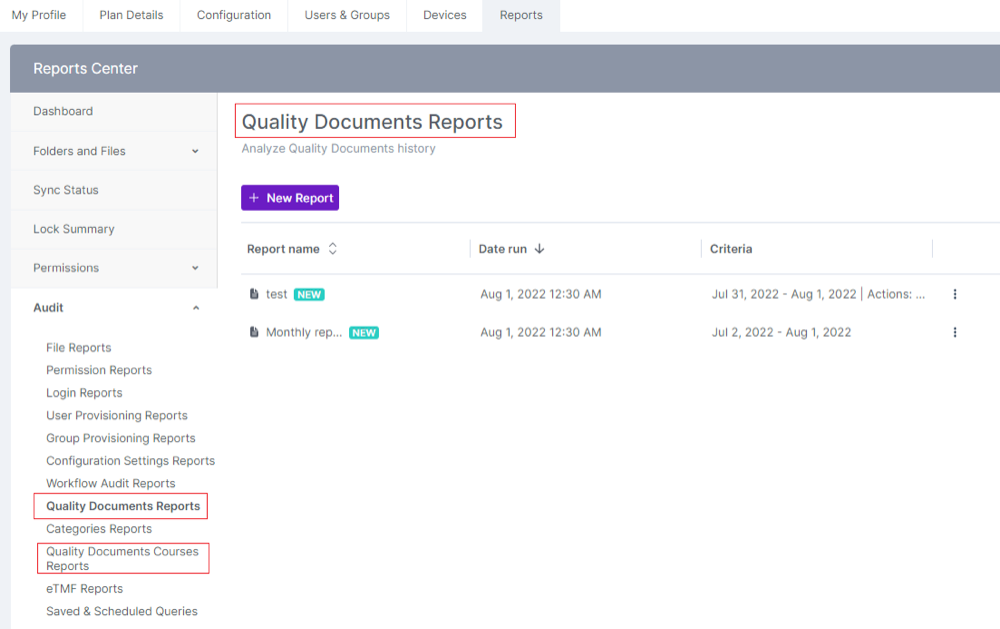
User Types & Roles:
Quality will be renamed to Controlled Documents.